Corrosion Protection
Transportation infrastructure elements across all sectors are intended to last for decades after installment, with as little time, effort, and money spent on maintenance as possible. Hot-dip galvanized steel supports this goal by providing superior barrier and cathodic protection, aided by the development of a zinc patina, to protect the steel within for generations with little or no maintenance.

Barrier Protection
The first line of corrosion defense is barrier protection. Like paints, the hot-dip galvanized coating provides barrier protection by isolating the steel from the electrolytes in the environment. As long as the barrier is intact, the steel is protected and corrosion will not occur. However, if the barrier is breached, corrosion will begin.
The impervious nature of zinc makes it a very good barrier coating. Steel items scattered throughout the airfield, such as ground support equipment (GSE) and jet bridges at local, regional, and international airports are not only exposed to all types of weather, but also to jet fuel fumes and other contaminants. Coatings such as paint that have pin holes in their barrier coating are susceptible to penetration by these elements and underfilm corrosion can spread rapidly. HDG's zinc coating is tightly bonded to the steel (3600 psi) and provides impervious, uniform protection providing no weak spots for corrosive elements to attack.
Cathodic Protection
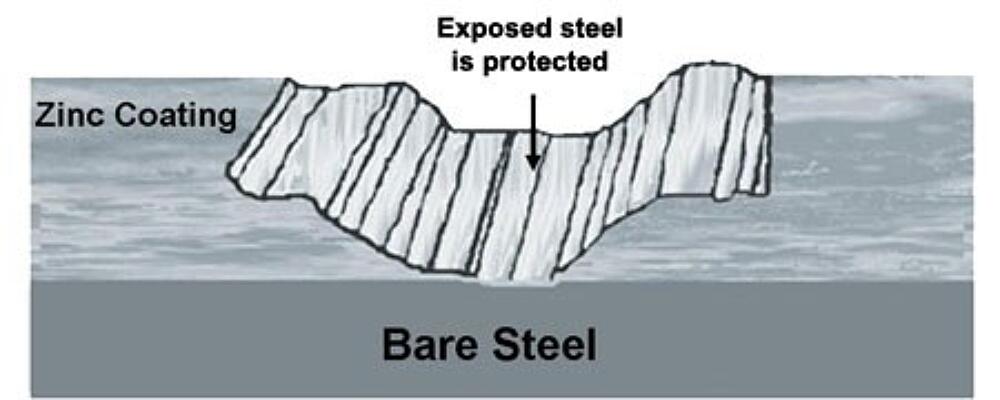
In addition to barrier protection, hot-dip galvanizing protects steel cathodically, which means zinc will preferentially corrode to protect the underlying base steel.

The Galvanic Series of Metals (left) is a list of metals arranged in order of electrochemical activity in seawater (the electrolyte). This arrangement of metals determines what metal will be the anode and cathode when the two are put in an electrolytic cell. Metals higher on the list are anodic to the metals below them meaning they provide cathodic or sacrificial protection when the two are connected. Therefore, zinc protects steel.
Galvanizings cathodic protection provides a second level of corrosion resistance for transportation infrastructure, especially for items along the highway and rail lines, as well as rail/bus stations. The sacrificial action of zinc means even if the coating is damaged by abrasive elements such as road debris, vehicle or bike contact, etc., no corrosion will begin until all of the surrounding zinc is consumed.
The natural cathodic protection of hot-dip galvanizing limits the need for maintenance of structures often constructed as install and forget it elements, which is important especially in mass transit where reliable service and safety is critical for the large volume of passengers.
Zinc Patina
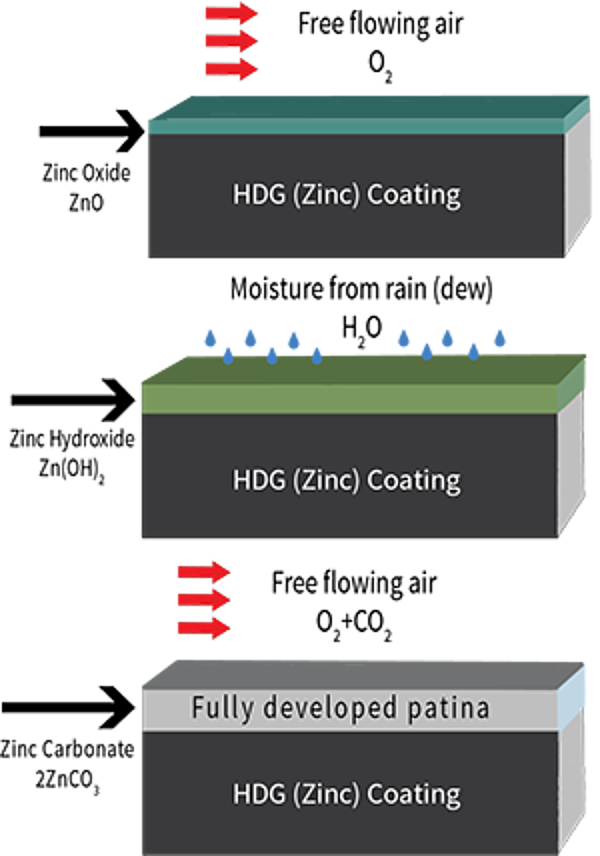
And the final factor in HDGs long-lasting corrosion protection is the development of the zinc patina. The zinc patina is the formation of zinc corrosion byproducts on the surface of the steel. Zinc, like all metals, begins to corrode when exposed to the atmosphere. As galvanized coatings are exposed to both moisture and free flowing air, corrosion byproducts will naturally form on the coating surface. The formation of these byproducts (zinc oxide, zinc hydroxide, and zinc carbonate) occurs during natural wet and dry cycles in the environment. The zinc patina, once fully developed, slows the corrosion rate of zinc to about 1/30th the rate of steel in the same environment and acts as an additional passive, impervious barrier for the hot-dip galvanized coating.