What is Hot-Dip Galvanizing?
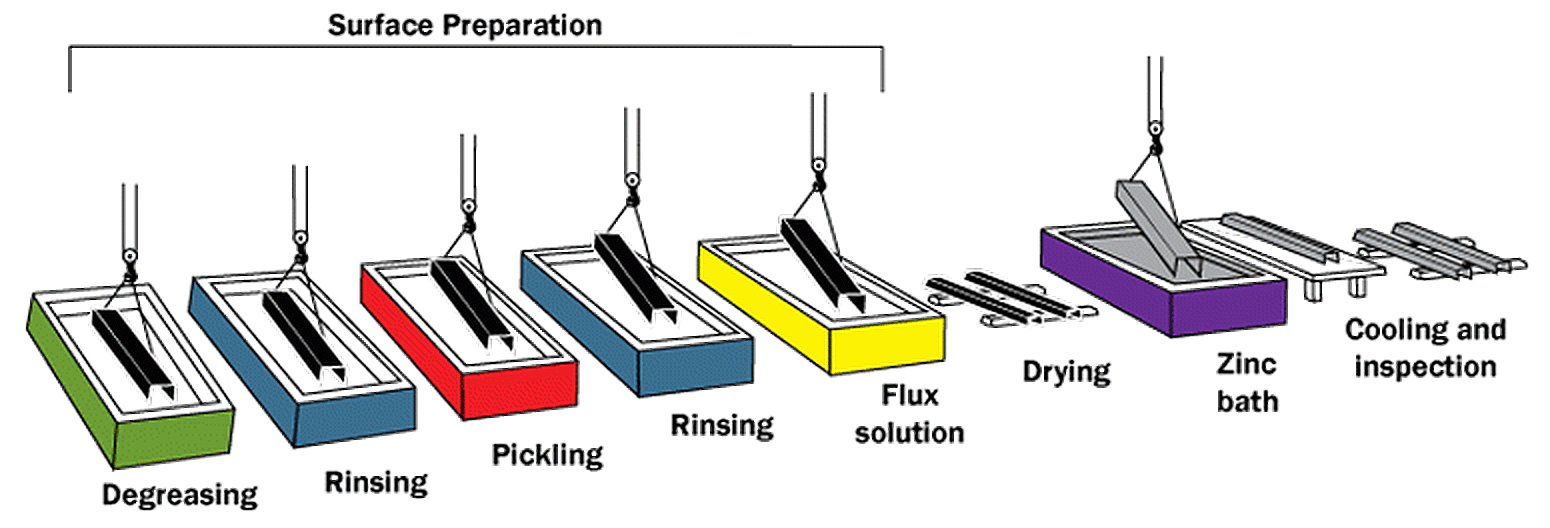
Hot-dip galvanizing (HDG) is the process of coating fabricated steel by immersing it in a bath of molten zinc. There are three fundamental steps in the hot-dip galvanizing process; surface preparation, galvanizing, and inspection (Figure 1).
Surface Preparation
When the fabricated steel arrives at the galvanizing facility, it is hung by wire or placed in a racking system which can be lifted and moved through the process by overhead cranes. The steel then goes through a series of three cleaning steps; degreasing, pickling, and fluxing. Degreasing removes dirt, oil, and organic residues, while the acidic pickling bath will remove mill scale and iron oxide. The final surface preparation step, fluxing, will remove any remaining oxides and coat the steel with a protective layer to prevent any further oxide formation prior to galvanizing. Proper surface preparation is critical, as zinc will not react with unclean steel.
Galvanizing
After surface preparation, the steel is dipped in the molten (830 F) bath of at least 98% zinc. The steel is lowered into the kettle at an angle that allows air to escape from tubular shapes or other pockets, and the zinc to flow into, over, and through the entire piece. While immersed in the kettle, the iron in the steel metallurgically reacts with the zinc to form a series of zinc-iron intermetallic layers and an outer layer of pure zinc.
Inspection
The final step is an inspection of the coating. A very accurate determination of the quality of the coating can be achieved by a visual inspection, as zinc does not react with unclean steel, which would leave an uncoated area on the part. Additionally, a magnetic thickness gauge can be used to verify the coating thickness complies with specification requirements.
Coating Benefits
Hot-dip galvanizing provides a number of benefits to the steel it protects. The metallurgically-bonded zinc-iron alloy layers not only create a barrier between the steel and the environment, but also cathodically protect the steel. The cathodic protection offered by zinc means the galvanized coating sacrifices itself to protect the underlying base steel from corrosion.
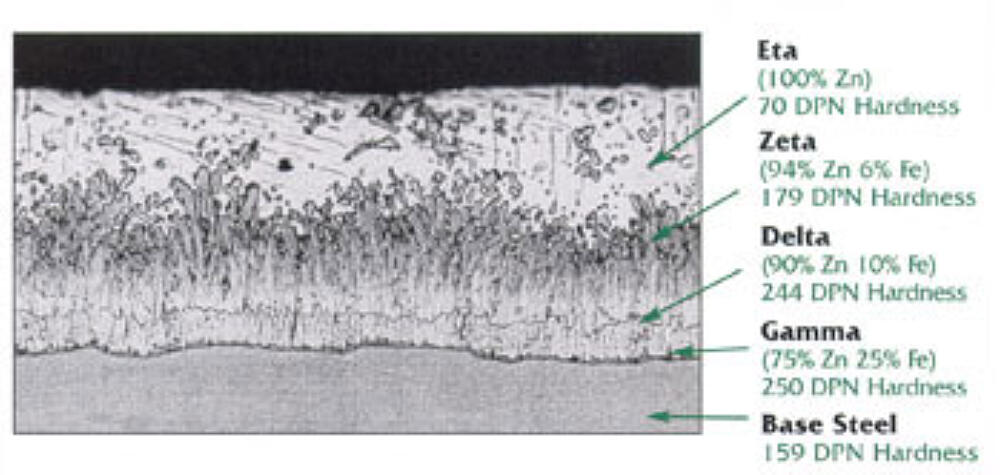
The tightly adhered coating, which has bond strength of around 3,600 psi, is also extremely abrasion-resistant, as the intermetallic layers are harder than the base steel (Figure 2). However, even if the coating is damaged, zincs sacrificial action will protect exposed steel up to ¼ inch away.
In addition to the cathodic protection offered by hot-dip galvanizing, there are a few other characteristics of the coating which provide longevity. First, reaction in the galvanizing kettle is a diffusion process, which means the coating grows perpendicular to the surface, ensuring all corners and edges have at least equal thickness to flat surfaces. Furthermore, the complete immersion in the zinc bath provides total coverage of the steel, including the interior of hollow structures.
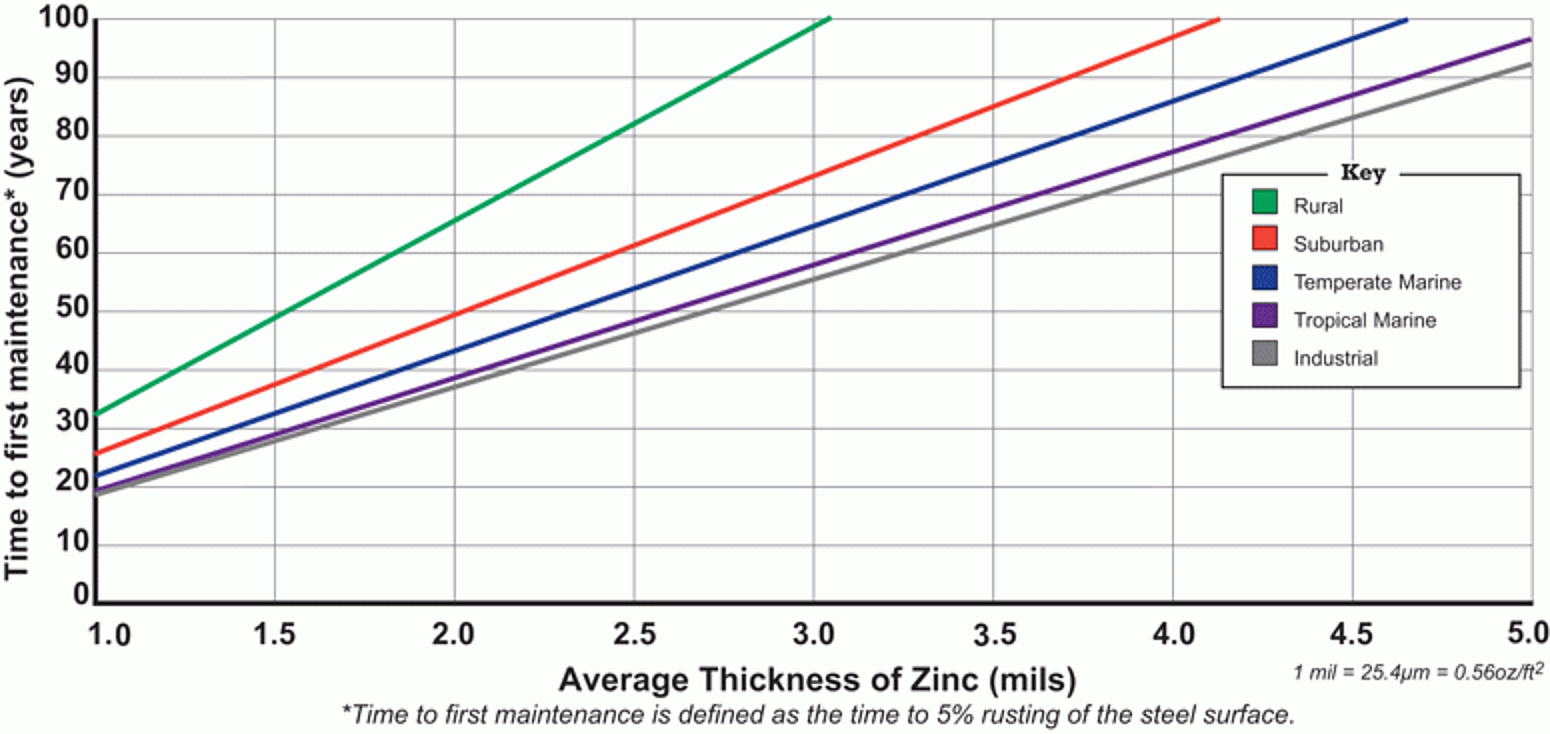
Finally, the zinc coating naturally develops an impervious layer of corrosion products on the surface, know as the zinc patina. The patina, cathodic protection, complete coverage and all of these other features, provide hot-dip galvanized steel with a long, maintenance-free service life. Thjavascript:void(null);e time to first maintenance for hot-dip galvanized steel can be seen in Figure 3.