What is the HDG Process?
The hot-dip galvanizing (HDG) process consists of three basic steps:
- Surface Preparation
- Galvanizing
- Inspection
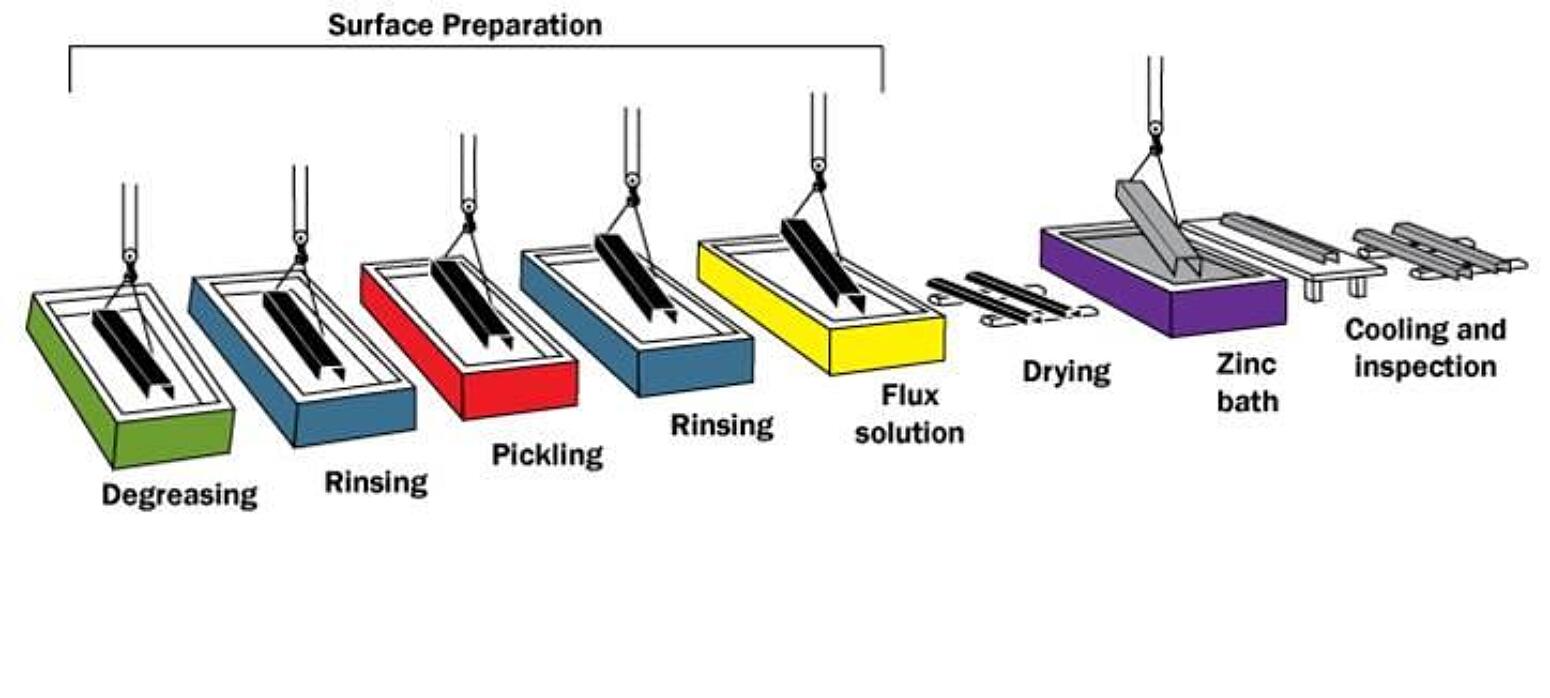
Surface Preparation
Surface preparation is a critical step in the application of any coating. In most instances where a coating fails before the end of its expected service life, it is because of incorrect or inadequate surface preparation. The galvanizing process has its own built-in means of quality control because zinc will not react with an unclean steel surface. Any failures or inadequacies in surface preparation will be immediately apparent when the steel is withdrawn from the zinc bath because the unclean areas will remain uncoated, and immediate corrective action can be taken.

Surface preparation for galvanizing consists of three steps:
Degreasing/Caustic Cleaning
A hot alkali solution, mild acidic bath, or biological cleaning bath removes organic contaminants such as dirt, paint markings, grease, and oil from the metal surface. Epoxies, vinyls, asphalt, or welding slag, which cannot be removed by degreasing, must be removed before galvanizing by grit-blasting, sand-blasting, or other mechanical means.
Pickling
A dilute solution of heated sulfuric acid or ambient hydrochloric acid removes mill scale and iron oxides (rust) from the steel surface. As an alternative to or in conjunction with pickling, this step can also be accomplished using abrasive cleaning or air blasting sand, metallic shot, or grit onto the steel.
Fluxing
The final surface preparation step in the galvanizing process, a zinc ammonium chloride solution, serves two purposes. It removes any remaining oxides and deposits a protective layer on the steel to prevent any further oxides from forming on the surface prior to immersion in the molten zinc.
Galvanizing

During the true galvanizing step of the process, the material is completely immersed in a bath of molten zinc. The bath chemistry is specified by ASTM B6, and requires at least 98% pure zinc maintained at 815-850 F (435-455 C).
While immersed in the kettle, the zinc reacts with the iron in the steel to form a series of metallurgically bonded zinc-iron intermetallic alloy layers, commonly topped by a layer of impact-resistant pure zinc.
Once the fabricated items' coating growth is complete, it is withdrawn slowly from the galvanizing bath, and the excess zinc is removed by draining, vibrating, and/or centrifuging.
The metallurgical reaction will continue after the materials are withdrawn from the bath, as long as it remains near bath temperature. Galvanized articles are cooled either by immersion in a passivation solution or water or by being left in open air.

Inspection
The inspection of hot-dip galvanized steel is simple and quick. The two properties of the hot-dip galvanized coating most closely scrutinized are coating thickness and appearance/surface condition. A variety of simple physical tests can be performed to determine thickness, uniformity, adherence, and appearance.
Products are galvanized according to long-established, accepted, and approved standards of ASTM, the International Standards Organization (ISO), the Canadian Standards Association (CSA), and the American Association of State Highway and Transportation Officials (AASHTO). These standards cover everything from the minimum coating thicknesses required for various categories of galvanized items to the composition of the zinc metal used in the process.
Testing methods and interpretation of results can be found in the inspection section of this site, as well as in the Inspection Course, and Inspection of Hot-Dip Galvanized Steel Products.

