Galvanizing

Once the steel has been completely cleaned, it is ready for immersion in the zinc bath. The galvanizing kettle contains zinc specified to ASTM B6, a document that specifies any one of three grades of zinc that are each at least 98% pure. Sometimes other metals may be added to the kettle to promote certain desirable properties in the galvanized coating.
The galvanizing kettle (Figure 5), is heated to a temperature ranging from 820-860 F (438-460 C), at which point the zinc is in a liquid state. The steel products are lowered into the galvanizing kettle at an angle, and stay in the bath until the steel heats to the bath temperature. Once the diffusion reaction of iron and zinc is complete, the steel product is withdrawn from the zinc kettle. The entire dip usually lasts less than ten minutes, depending upon the thickness of the steel.
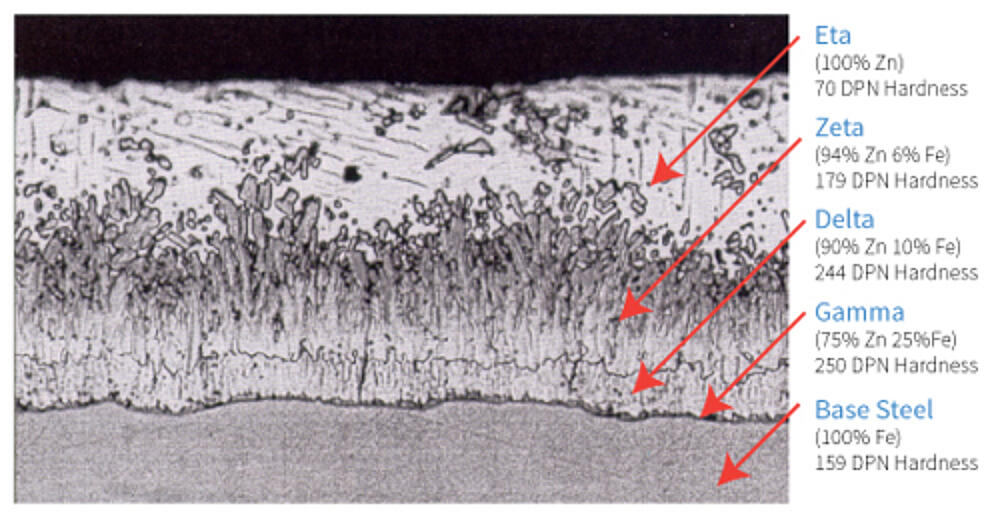
The below coating structure, as seen in Figure 6, is typical for steels of recommended chemistry for galvanizing where the thickness of the coating is limited by the interdiffusion of iron and zinc.

Post-Treatment
When the steel is removed from the galvanizing kettle, it may receive a post-treatment to enhance the galvanized coating. One of the most commonly used treatments is quenching. The quench tank contains mostly water but may also have chemicals added to create a passivation layer that protects the galvanized steel during storage and transportation. Other finishing steps include removal of zinc drips or spikes, by grinding them off.