Galvanizing Process
Hot-dip galvanizing is the process of immersing iron or steel in a bath of molten zinc to produce a corrosion resistant, multi-layered coating of zinc-iron alloy and zinc metal. While the steel is immersed in the zinc, a metallurgical reaction occurs between the iron in the steel and the molten zinc. This reaction is a diffusion process, so the coating forms perpendicular to all surfaces creating a uniform thickness throughout the part.
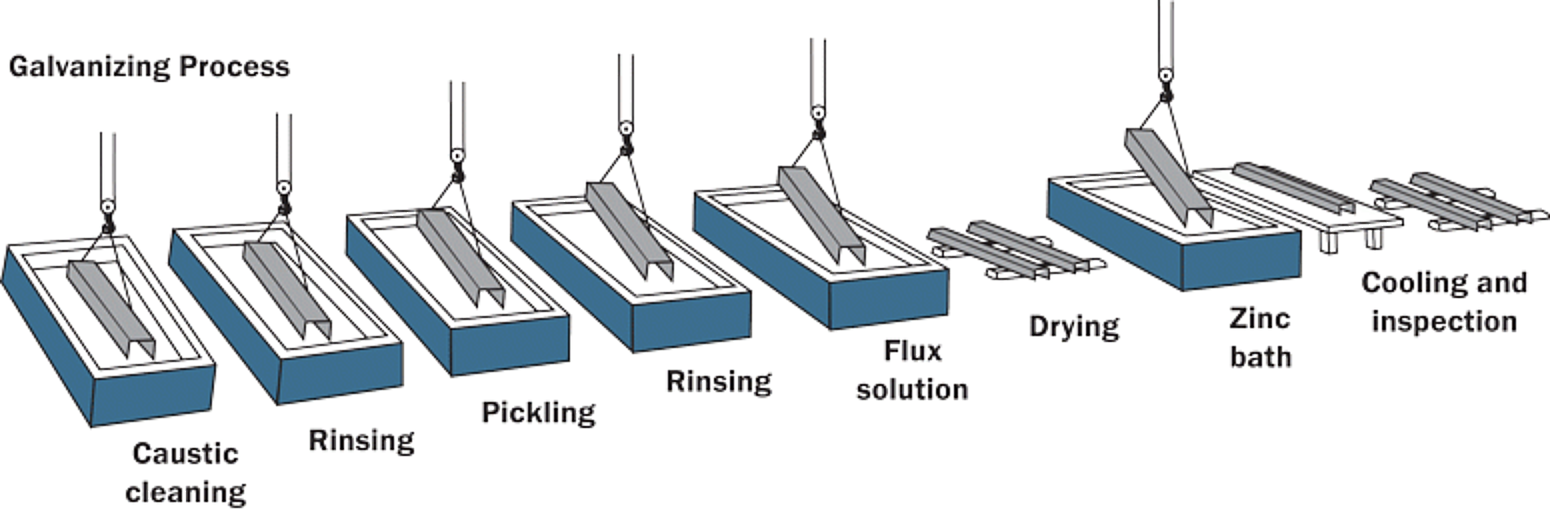
The hot-dip galvanizing process (Figure 1) has been used since 1742, providing long-lasting, maintenance-free corrosion protection at a reasonable cost for decades. Although hot-dip galvanizing has been utilized to protect steel for generations, the galvanizing process continues to evolve with new technologies and creative chemistries. The three main steps in the hot-dip galvanizing process are surface preparation, galvanizing, and post-treatment, each of which will be discussed in detail. The process is inherently simple, which is a distinct advantage over other corrosion protection methods.

Figure 2 shows a series of steel structures with visible evidence of corrosion. Rust and corrosion are expensive for owners and taxpayers. Deteriorating buildings, roads, bridges, etc. are costly to repair, and without sufficient corrosion protection, maintenance is done often, or in the worst cases, the structure must be rebuilt. With the push toward sustainable development, specifying structures with longevity that require little maintenance over time provide both environmental and economic benefits.