Hot-Dip Galvanizing (HDG)
Whether an artful sculpture glinting under the sun or a sturdy bridge arching over a rushing river, hot-dip galvanizing protects steel from corrosion for generations throughout the world. New technologies and creative chemistry continue to evolve this 150-year old process, which facilitates its continued use in myriad applications as well as innovative ideas for new ones.
Before we get into the details of hot-dip galvanizing's benefits, it is important to understand how the coating is applied. The galvanizing process consists of three basic steps: surface preparation, galvanizing, and inspection.
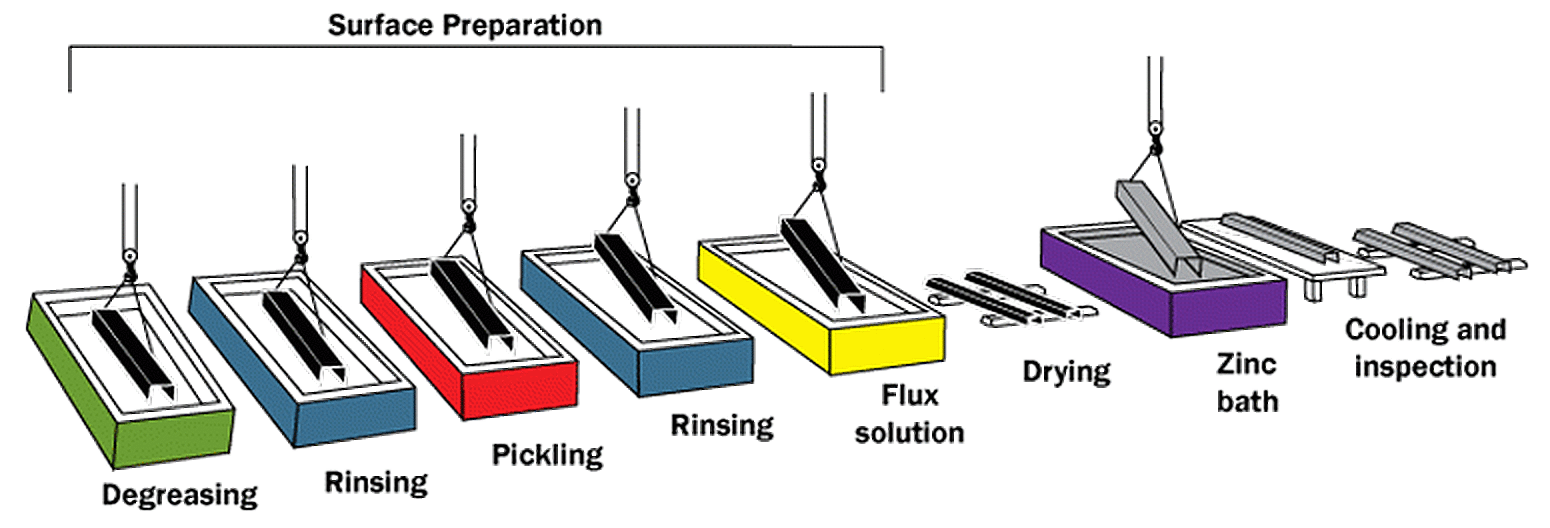
Surface Preparation

Surface preparation is a critical step in the application of any coating. In most instances where a coating fails before the end of its expected service life, it is because of incorrect or inadequate surface preparation.
The surface preparation step in the galvanizing process has its own built-in means of quality control because zinc simply will not react with unclean steel. Any failures or inadequacies in surface preparation will be immediately apparent when the steel is withdrawn from the zinc bath because the unclean areas will remain uncoated, and immediate corrective action can be taken.
Surface preparation consists of three steps:
- Degreasing - A hot alkali solution, mild acidic bath, or biological cleaning bath removes organic contaminants such as dirt, paint markings, grease, and oil from the metal surface. Epoxies, vinyls, asphalt, or welding slag, which cannot be removed by degreasing, must be removed before galvanizing by grit-blasting, sand-blasting, or other mechanical means.
- Pickling - A dilute solution of heated sulfuric acid or ambient hydrochloric acid removes mill scale and iron oxides (rust) from the steel surface. As an alternative to or in conjunction with pickling, this step can also be accomplished using abrasive cleaning or air blasting sand, metallic shot, or grit onto the steel.
- Fluxing - The final surface preparation step, a zinc ammonium chloride solution, serves two purposes. It removes any remaining oxides and deposits a protective layer on the steel to prevent any further oxides from forming on the surface prior to immersion in the molten zinc.
Galvanizing

The “galvanizing” step of the process occurs when steel is completely immersed in a bath (kettle) of molten zinc. According to specification, the bath chemistry must be at least 98% pure zinc and maintained at a temperature approximately 840 F (449 C). The steel is lowered at an angle by crane hoist. This allows air to escape from tubular shapes or pockets that may be within the design of a fabricated piece and of course permits the molten zinc to displace the air.
While immersed in the kettle, the zinc reacts with iron in the steel to form a series of zinc-iron inter-metallic alloy layers. Once the fabricated item reaches bath temperature the coating growth is complete, and the articles are withdrawn slowly from the galvanizing bath. Excess zinc is removed by draining, vibrating, and/or centrifuging. The metallurgical reaction will continue after withdrawal from the bath, as long as the article remains near bath temperature. Articles are cooled either by immersion in a passivation solution or water or by being left in open air.
Inspection

The last phase of the process, inspection, is simple and quick. The two properties of the hot-dip galvanized coating closely scrutinized are coating thickness and coating appearance. There are additional tests outlined for adherence, but these are typically only administered as a “referee” test, or when an issue is suspected.
A very accurate determination as to the quality of the galvanized coating can be accomplished through a visual inspection of the material, because as stated earlier zinc will not react with unclean steel. A variety of simple physical and laboratory tests may also be performed to determine thickness, uniformity, adherence, and appearance to ensure the coating is in compliance with specification requirements. Products are galvanized according to long established, accepted, and approved standards of ASTM.